Dr. Md.Salamuddin
INTRODUCTION
It is the prime instinct of scientist to be curious to understand the natural phenomena occurring around him. Most researches are outcome of this curiosity. Coordination compounds such as chlorophyll hemoglobin, vitamin B12 etc. are acting as metalloenzyme. These have metal ions coordinated through nitrogen and oxygen so in order to understand a large amount of work has been appeared in literature on coordination compounds with nitrogen-oxygen donar ligands. Recently sulphur is the third element playing its role in natural phenomena.
RECOVERY OF COPPER
Copper was determined with salicyladoxime. 2n sodium hydroxide was added to the copper solution (100ml.), which contained a known weight of Cu compound, until a slight permanent precipitate was formed. It was dissolved in little dilute acetic acid. Salicyldoxime regent was added in slight excess at room temperature. Precipitated complex was filtered off on a weighed
sintered glass crucible, washed with water until the washing give no colour with ferric chloride, and dried to constant weight at 100-105 C (about one hour). It was weighed as Cu(C7H6O2N)2.
PREPRATION OF O-METHOXYBENZALIDINETHIOSEMICARBAZONE COPER (II)
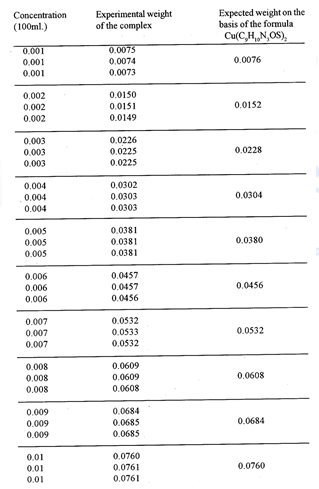
I gram CuSo4.5H2O was dissolved in distilled water and treated with 1.85 gram of the ligand dissolved in acetone.
The whole mass was digested on steam-bath for half an hour. The red-brown precipitate was filtered off and dried in air.
Found :
Cu………………………13.20%
C…………………………45.00%
H…………………………4.02%
N………………………..17.70%
Required for [Cu(C9H10N3OS)2:-
Cu………………………….13.24%
C……………………………45.05%
H……………………………4.17%
N…………………………..17.52%
The complex is insoluble in water but dissolves in acetone, ethanol and methanol. The compound does not loss any weight upto 120C, above this temperature the complex begins to decompose.
The complex is square planar as indicated by its µβ which is equal to 1.7327 B.M. The i.r. spectra26 of the complex indicate the following structure of the complex (Fig.1)
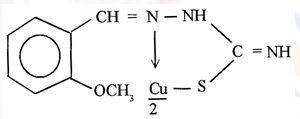
(Fig.1)
ESTIMATION OF CU(II) WITH O-METHOXYBENZALIDINETHIOSEMICRABAZONE
The reagent was prepared as in the case of Ni(II) estimation. The solutions of Cu(II) with varying concentration were used for the
Iodometric determination of the metal.
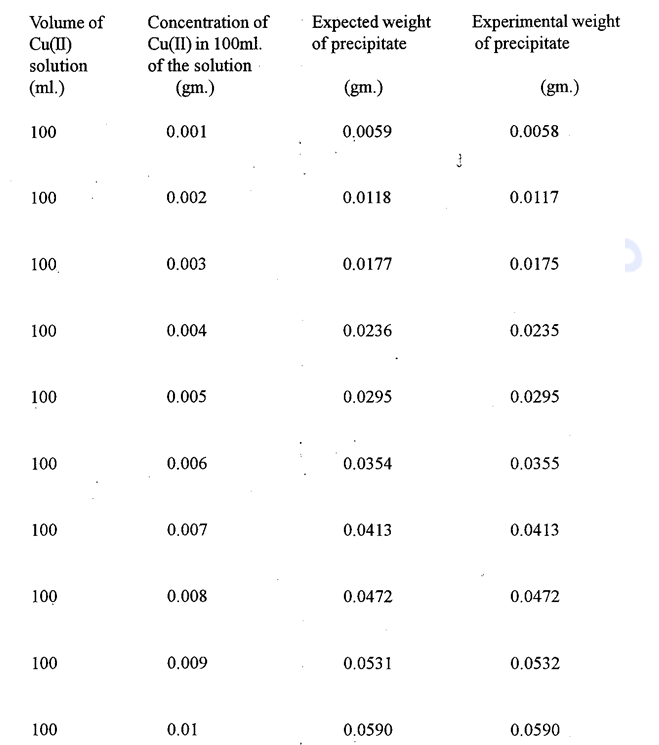
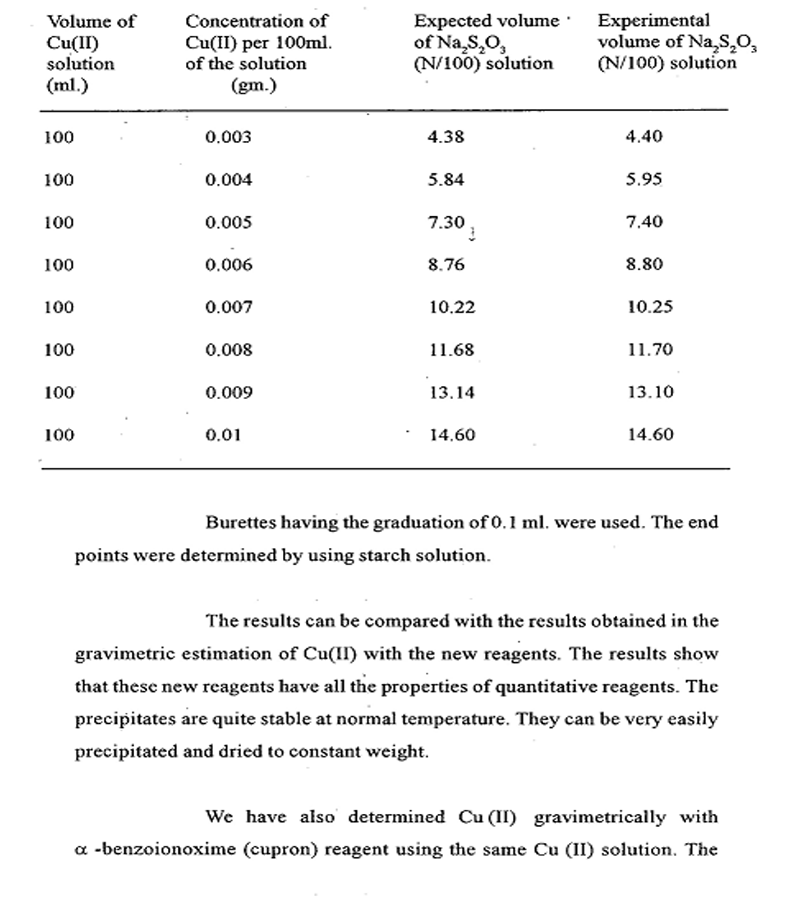
Result are tabulated below:-
ass was dije-sted on steam-bath for about half an hour and then filtered through a weighed sintered glass crucible. The precipitate was washed with hot ter ill from the acid ion and dried in an air oven to a constant weight at 120-130 C. Three such periments were performed for each concentration.
RESULT & DISCUSSION
Result are tabulated below:-
Thus the result obtained with the reagent are not so good in the low concentrations. Besides the reagents cannot be used in neutral medium. There agent is specific for copper only in ammoniacal medium.
On the other hand our new reagents are very susceptible for the precipitation of Cu(II) from the low concentration. The precipitates are very easy to filter and they can be dried at 110-1300C to constant weight.
The precipitate with α – benzoinoxime is green which may be confused with Cu(II) hydroxides. The precipitate obtained with the new regents are brown to black and hence the confusion is eliminated.
REFERENCES
(1) P.C.H. Mitchell and R.J.P. Williams., : J. chem..Soc., (1960) 1912.
(2) C.N.R. Rao, R. Venkataraghavan and T.R. Kasturi, Can. J. Chem., 42, (1964) 36.
(3) A.L. Castro and M.R. Truter, : J. Chem. Soc., (1963) 1309.
(4) K. Swamithan and H. lrving, : J. Inorg. Nucl.Chem. 26, (1964) 1291.
(5) R. Gronbaek and S.E. Rasmuseen, : Acta, Chem. Scand., 16, (1962) 2325.
(6) A. Braibanti, M.A. Pellinghelli, A. TiripicohioCamellin.: Inorg. Chem. Acta., 5, (1971) 523.
(7) C. Puglisi and R. Levitus., : J. Inorg. Nucl.Chem. 20, (1967) 1069.
Dr. Md. Salamuddin
Assistant Professor
B.M.A. College, Baheri, Darbhanga